PD-1(程序性死亡受体1)及其配体PD-L1(程序性死亡配体1)在肿瘤免疫逃逸中起着关键作用。PD-1/PD-L1通路通过抑制T细胞的活化和增殖,导致肿瘤细胞逃避免疫系统的攻击。因此,针对这一通路的免疫检查点抑制剂(如抗PD-1/PD-L1抗体)已成为多种癌症治疗的重要手段。然而,尽管PD-1/PD-L1抑制剂在某些患者中取得了显著的疗效,但只有少数患者能够获得持久地应答,许多患者对这些治疗表现出原发或获得性耐药[1.2]。
对PD-1耐药机制的深入理解至关重要,因为耐药不仅影响患者的预后,也增加了治疗的复杂性。因此,建立有效的PD-1耐药模型可以帮助我们明确耐药的生物学机制,为新疗法的开发和临床治疗策略的优化提供重要依据。这些模型的构建有助于研究不同种类的耐药机制以及探索联合治疗的可能性。
PD-1耐药机制
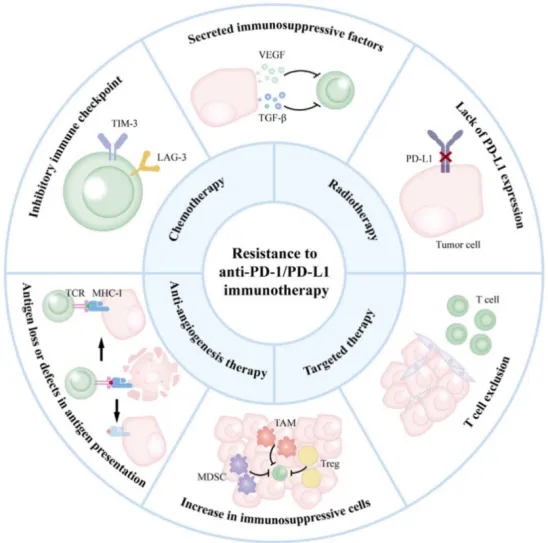
Mechanisms of resistance to anti-PD-1/PD-L1 immunotherapy. The primary mechanisms of resistance to anti-PD-1/PD-L1 therapy, including the lack of PD-L1 expression; T cell exclusion; increase in immunosuppressive cells; antigen loss or defects in antigen presentation; inhibitory immune checkpoint; secreted immunosuppressive factors. This also includes strategies for overcoming resistance and combining with anti-PD-L1 inhibitors, like combination with chemotherapy, radiotherapy, targeted therapy and anti-angiogenesis therapy.[1]
百奥赛图目前已构建多种PD-1耐药模型。这些PD-1耐药动物模型能帮助人们深入探索耐药机制,并适配多种不同的应用场景。
诱导的耐药细胞系
通过反复多次的PD-1抑制剂的处理,在B-hPD-1/hPD-L1 mice模型上,我们成功建立了PD-1耐药的MC38细胞株。这些细胞一方面表现出对PD-1抑制剂的降低敏感性;另一方面,耐药的细胞株免疫抑制的肿瘤微环境可能是其耐药的主要原因[3-5],TILs的流式分析结果显示,对PD-1给药正常响应的肿瘤组织中,T细胞总数,CD8+T细胞及MHCII+细胞的比例明显升高,而在诱导的PD-1耐药细胞系中,肿瘤微环境展现出很强的抑制性,包括MDSC及Th细胞的增多、CD8+T细胞比例的降低等。
诱导的PD-1耐药MC38
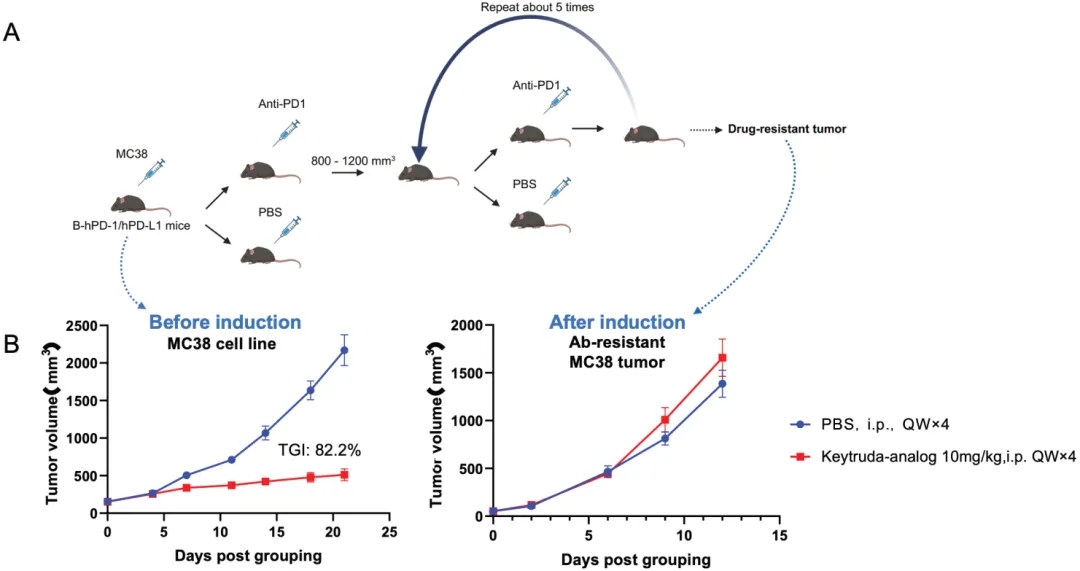
An induced hPD-1 antibody-resistant model was established. (A) An induced hPD-1 antibody-resistant model was established by multiple rounds of hPD-1 antibody retreatment on MC38-bearing humanized B-hPD-1/hPD-L1 mice. (B) The anti-tumor drug efficacy on MC38 cells before (TGl: 82.2%) and after induction validated the successful establishment of the hPD-1 antibody-resistant model.
PD-1-resistant MC38 和MC38的TILs分析
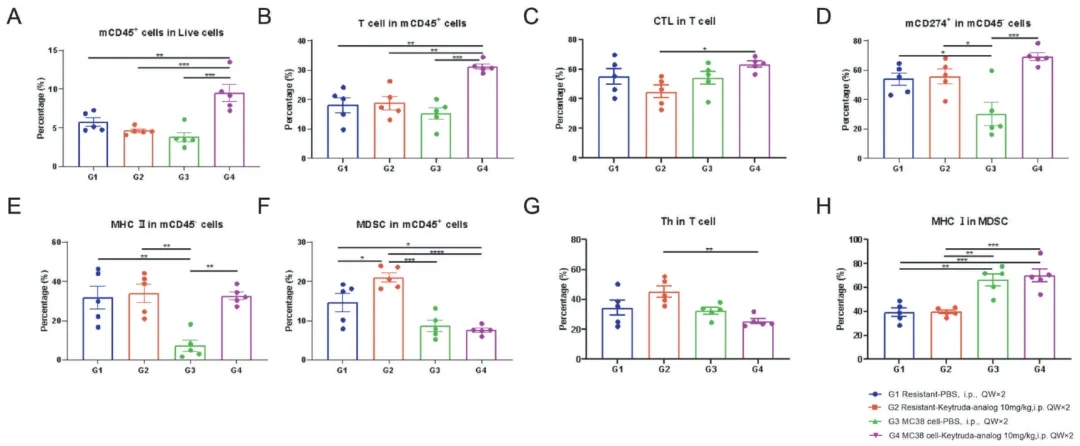
TILs profiling in hPD-1 antibody resistant animal models.Tumor tissues from indicated groups after treatment were collected and analyzed by flow cytometry at the end of the study. The specific immune cell subsets were quantified as in (A) mCD45+ cells in live cells. (B) CD3+ cells in mCD45+ cells. (C) CD8+ cells in CD3+ cells. (D) mCD274+ (mPD-L1) in mCD45+ cells. (E) MHCII in mCD45- cells. (F) MDSC in mCD45+ cells. (G) CD4+ cells in CD3+ cells. (H) MHCI in MDSCs. Data shown as mean ± SEM and analyzed using one-way ANOVA followed by Tukey's test comparing each group. ( *p<0.05, **p<0.01, ***p<0.001)
药效不敏感的肿瘤细胞系
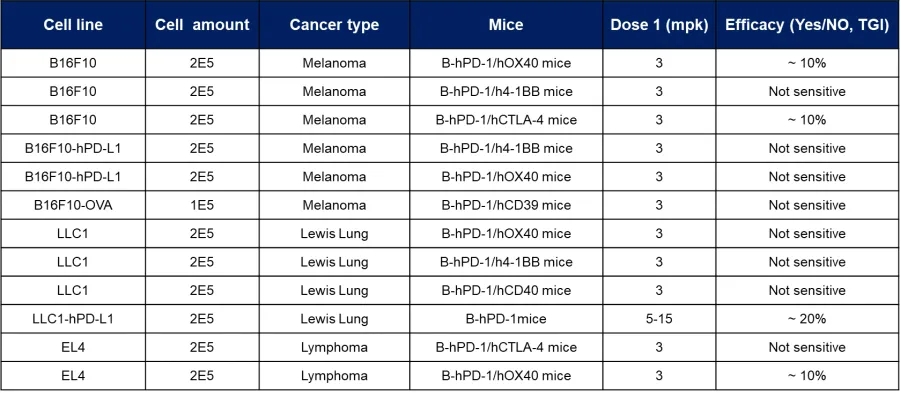
在正常情况下,有些肿瘤细胞系对PD-1抑制剂的反应相对不敏感。这些细胞可做为天然的PD-1耐药细胞系用于相关药效的评价,如黑色素瘤B16F10细胞系、肺癌LLC1细胞系、淋巴癌EL4细胞系等。这可能是由于这些细胞存在独特的信号通路或分子特征,使其对PD-1抑制剂无效。通过分析这些细胞系,我们能够识别耐药相关的生物标志物,并为未来的个体化治疗提供线索。
PD-1药效不敏感细胞系
药效不敏感的老年鼠
老年小鼠作为PD-1耐药模型的一种,展现出不同于年轻小鼠的免疫反应特征,治疗与非治疗组中相比,免疫细胞的比例基本不变,展现出一种不响应的肿瘤微环境。老年小鼠中肿瘤的免疫逃逸机制可能与衰老相关的免疫功能下降有关,同时也可能表现出特定的代谢变化。这些模型在评估新疗法或组合疗法的有效性上提供了重要的平台。
PD1耐药的老年鼠

mPD-1 antibody resistance was evaluated in aged wild-type mice. MC38 cells were inoculated in the young (7 weeks old) and aged (18 months old) C57BL/6 mice, respectively. Animals were intravenously administered either PBS or mPD-1 at a dose of 5mg/kg, twice weekly for six doses. Drug efficacy was determined by calculating the TGl between control and treatment groups. (A) Young animals displayed a TGI of 85.8%. (B) Aged animals displayed a TGl of 6%. Data shown as mean ± SEM.
老年鼠和幼年鼠中MC38的TILs分析
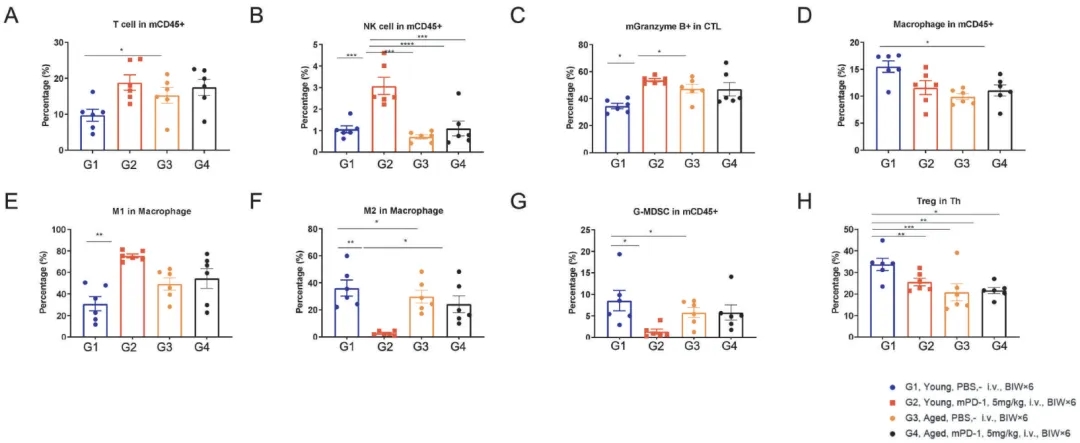
TILs profiling in mPD-1 antibody resistant animal models. Tumor tissues from indicated groups after treatment were collected and analyzed with FACS at the end of the study. The specific immune cell subsets were quantified as in (A) CD3+T cells in mCD45+ cells. (B) NK cells in mCD45+ cells. (C) mGranzyme B+ in mCD8+ cells. (D) Macrophage in mCD45+ cells. (E) M1 in Macrophage. (F) M2 in Macrophage. (G) G-MDSC in mCD45+ cells. (H) FOXP3+ Treg cells in mCD3+ CD4+ cells. Data shown as mean ± SEM and analyzed using one-way ANOVA followed by Tukey's test comparing each group. (*p<0.05, **p<0.01, ***p<0.001)
PD-1耐药模型在临床前研究中具有广泛的应用前景,如耐药机制的研究、不同生物背景下的个性化治疗策略探索、新药研发及新型免疫疗法的联合应用测试等[6.7]。综上所述,建立和优化PD-1耐药模型具有重要的科学研究价值和临床转化潜力。
参考文献:
[1] Hu L, Sun C, Yuan K, Yang P. Expression, regulation, and function of PD-L1 on non-tumor cells in the tumor microenvironment. Drug Discov Today. 2024 Sep 13;29(11):104181.
[2] Chen, D., Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 541, 321–330 (2017).
[3] Aksoylar, HI., Boussiotis, V.A. PD-1+ Treg cells: a foe in cancer immunotherapy?. Nat Immunol 21, 1311–1312 (2020).
[4] Murciano-Goroff, Y.R., Warner, A.B. & Wolchok, J.D. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res 30, 507–519 (2020).
[5] Wang Q, Xie B, Liu S, Shi Y, Tao Y, Xiao D, Wang W. What Happens to the Immune Microenvironment After PD-1 Inhibitor Therapy? Front Immunol. 2021 Dec 23;12:773168.
[6] Liu, B., Zhou, H., Tan, L. et al. Exploring treatment options in cancer: tumor treatment strategies. Sig Transduct Target Ther 9, 175 (2024).
[7] Ni, Jj., Zhang, Zz., Ge, Mj. et al. Immune-based combination therapy to convert immunologically cold tumors into hot tumors: an update and new insights. Acta Pharmacol Sin 44, 288–307 (2023).






 010-56967680
010-56967680 info@bbctg.com.cn
info@bbctg.com.cn 苏公网安备:32068402320845号
苏公网安备:32068402320845号