中文
B-hTLR8/hTLR7 mice
| Strain Name |
C57BL/6-Tlr8tm1(TLR8)Bcgen Tlr7tm1(TLR7)Bcgen/Bcgen |
Common Name | B-hTLR8/hTLR7 mice |
| Background | C57BL/6 | Catalog number | 121169 |
| Aliases |
TLR8, CD288, IMD98 |
||
模型验证
mRNA expression analysis
Protein expression analysis in monocytes
Protein expression analysis in macrophages
Protein expression analysis in DCs
Analysis of leukocytes cell subpopulation in spleen
Analysis of T cell subpopulation in spleen
Analysis of leukocytes cell subpopulation in blood
Analysis of T cell subpopulation in blood
Analysis of leukocytes cell subpopulation in lymph node
Analysis of T cell subpopulation in lymph node
Analysis of mTNF-α and mIFN-γ protein expression by ELISA
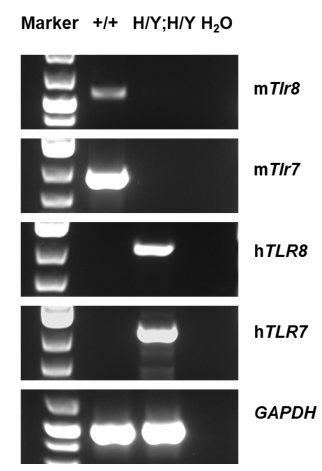
Strain specific analysis of TLR8 and TLR7 gene expression in wild-type C57BL/6 mice and homozygous B-hTLR8/hTLR7 mice by RT-PCR. Mouse Tlr8 and Tlr7 mRNA was detectable in wild-type C57BL/6 mice (+/+). Human TLR8 and TLR7 mRNA was detectable only in homozygous B-hTLR8/hTLR7 mice but not in wild-type mice.
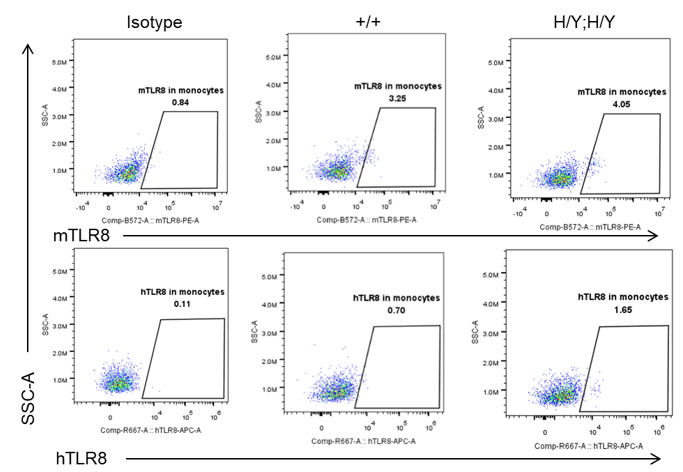
Strain specific TLR8 expression analysis in homozygous B-hTLR8/hTLR7 mice by flow cytometry. Splenocytes were collected from wild-type mice (+/+) and homozygous B-hTLR8/hTLR7 mice (H/Y;H/Y), and analyzed by flow cytometry with species-specific anti-TLR8 antibody. Mouse TLR8 was detectable in wild-type mice and homozygous B-hTLR8/hTLR7 mice due to the anti-mouse TLR8 antibody cross-reacts with human TLR8. Human TLR8 was exclusively detectable in homozygous B-hTLR8/hTLR7 mice but not WT mice.
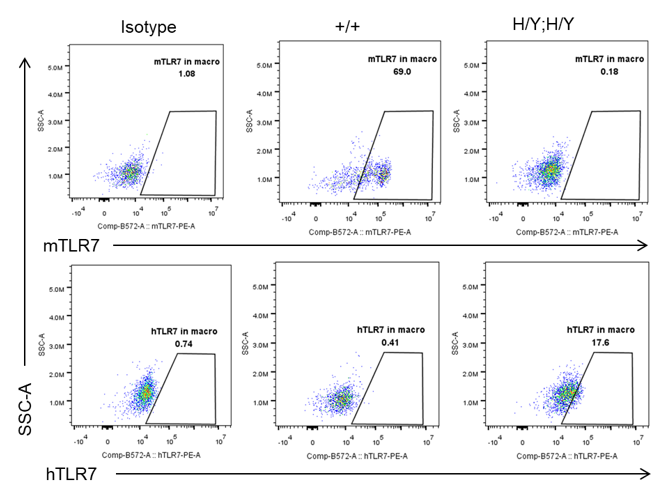
Strain specific TLR7 expression analysis in homozygous B-hTLR8/hTLR7 mice by flow cytometry. Splenocytes were collected from wild-type mice (+/+) and homozygous B-hTLR8/hTLR7 mice (H/Y;H/Y), and analyzed by flow cytometry with species-specific anti-TLR7 antibody. Mouse TLR7 was exclusively detectable in WT mice but not in homozygous B-hTLR8/hTLR7 mice. Human TLR7 was exclusively detectable in homozygous B-hTLR8/hTLR7 mice but not WT mice.
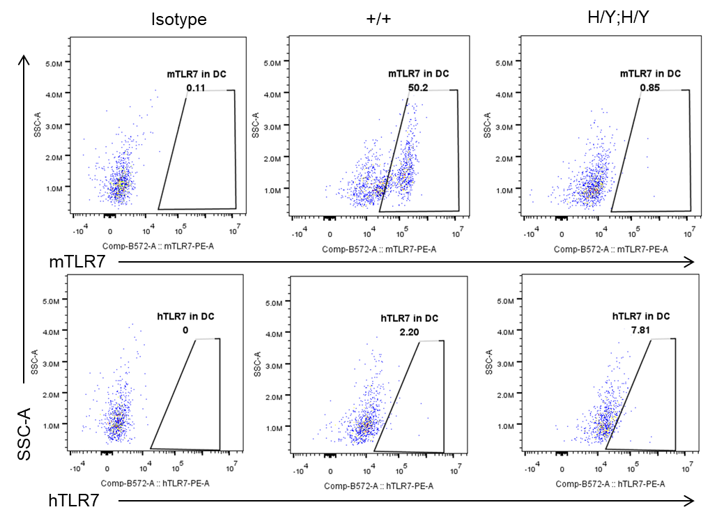
Strain specific TLR7 expression analysis in homozygous B-hTLR8/hTLR7 mice by flow cytometry. Splenocytes were collected from wild-type mice (+/+) and homozygous B-hTLR8/hTLR7 mice (H/Y;H/Y), and analyzed by flow cytometry with species-specific anti-TLR7 antibody. Mouse TLR7 was exclusively detectable in WT mice but not in homozygous B-hTLR8/hTLR7 mice. Human TLR7 was exclusively detectable in homozygous B-hTLR8/hTLR7 mice but not WT mice.
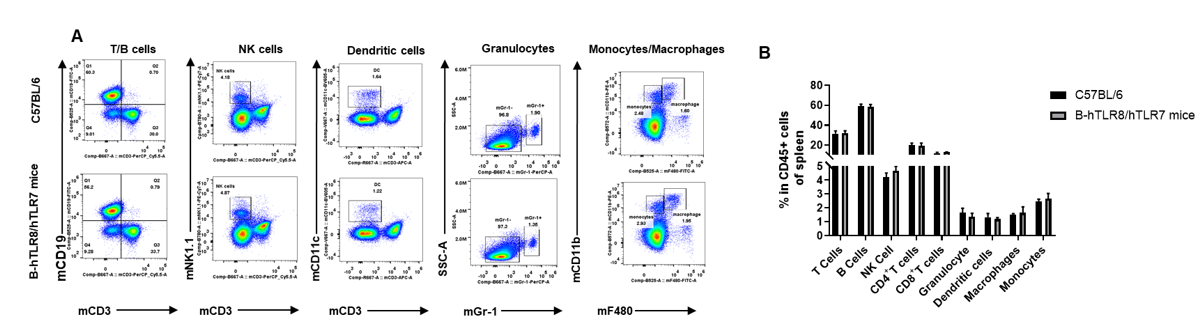
Analysis of spleen leukocyte subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hTLR8/hTLR7 mice (n=3, 8-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-hTLR8/hTLR7 mice were similar to those in the C57BL/6 mice, demonstrating that TLR8 humanized does not change the overall development, differentiation or distribution of these cell types in spleen.
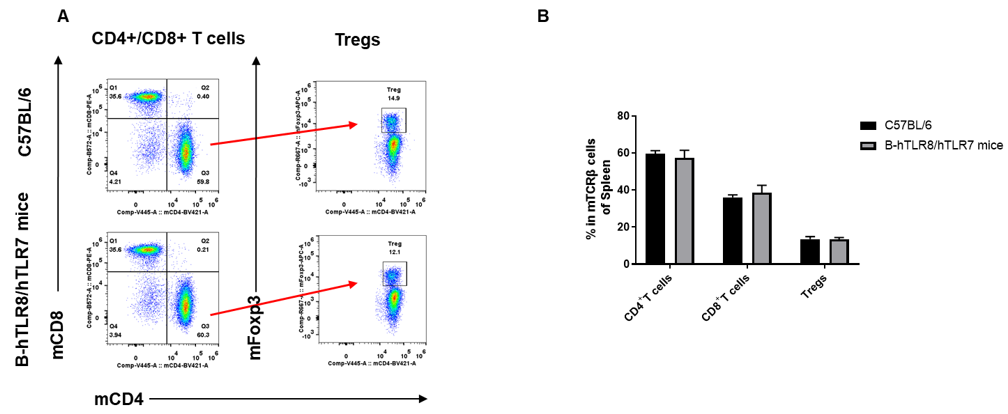
Analysis of spleen T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hTLR8/hTLR7 mice (n=3, 8-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for TCRβ+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD8+ T cells , CD4+ T cells and Tregs in homozygous B-hTLR8/hTLR7 mice were similar to those in the C57BL/6 mice, demonstrating that TLR8 humanized does not change the overall development, differentiation or distribution of these cell types in spleen.
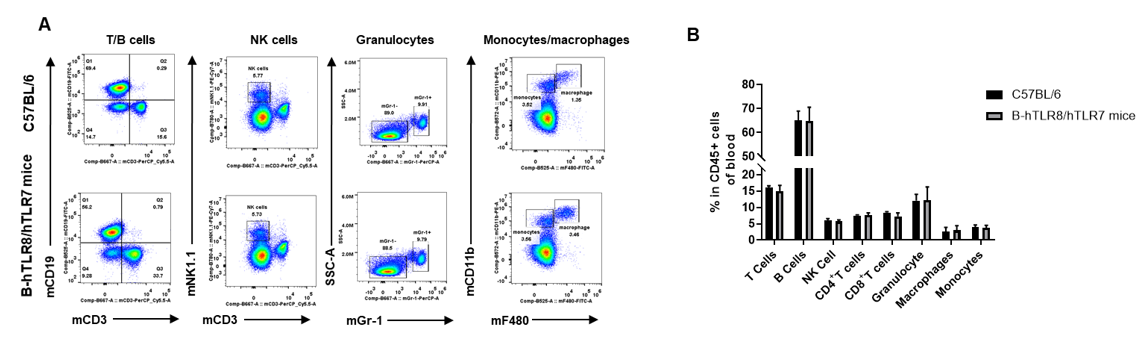
Analysis of blood leukocyte subpopulations by FACS. Blood were isolated from female C57BL/6 and B-hTLR8/hTLR7 mice (n=3, 8-week-old). Flow cytometry analysis of the blood was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-hTLR8/hTLR7 mice were similar to those in the C57BL/6 mice, demonstrating that TLR8 humanized does not change the overall development, differentiation or distribution of these cell types in blood.
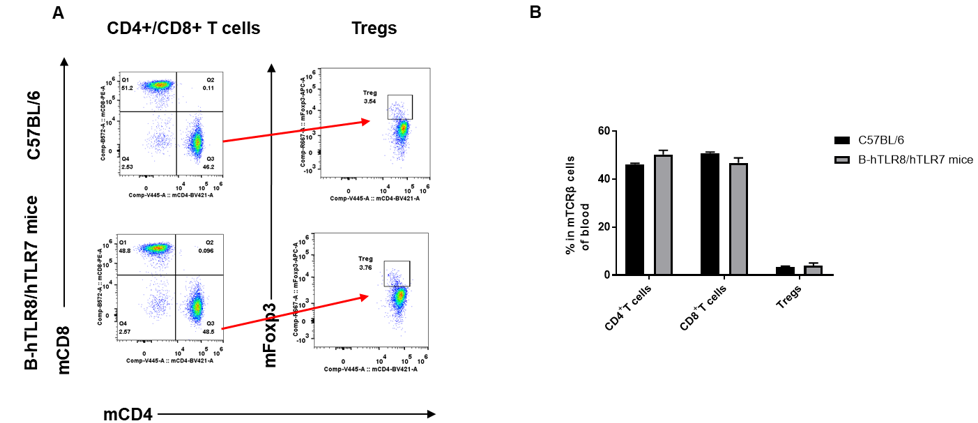
Analysis of blood T cell subpopulations by FACS. Blood were isolated from female C57BL/6 and B-hTLR8/hTLR7 mice (n=3, 8-week-old). Flow cytometry analysis of the blood was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for CD3+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD4+ T cells, CD8+ T cells, and Tregs in homozygous B-hTLR8/hTLR7 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hTLR8 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in blood.
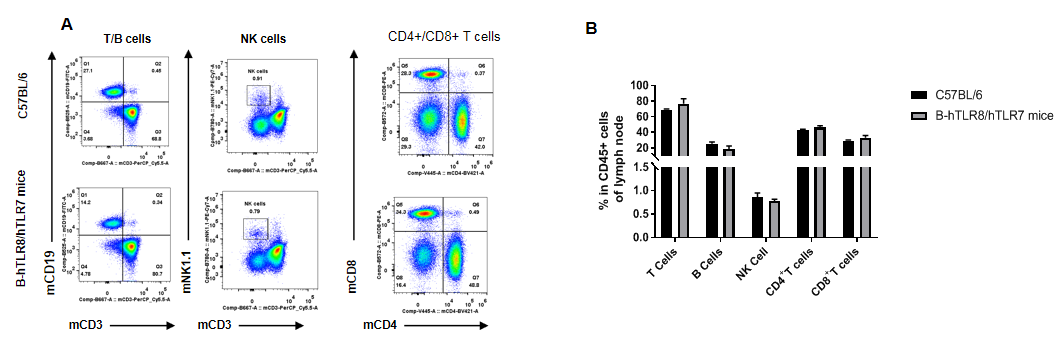
Analysis of lymph node leukocyte subpopulations by FACS. Lymph node cells were isolated from female C57BL/6 and B-hTLR8/hTLR7 mice (n=3, 8-week-old). Flow cytometry analysis of the cells was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells,CD4+ T cells,CD8+ T cells in homozygous B-hTLR8/hTLR7 mice were similar to those in the C57BL/6 mice, demonstrating that TLR8 humanized does not change the overall development, differentiation or distribution of these cell types in lymph node.
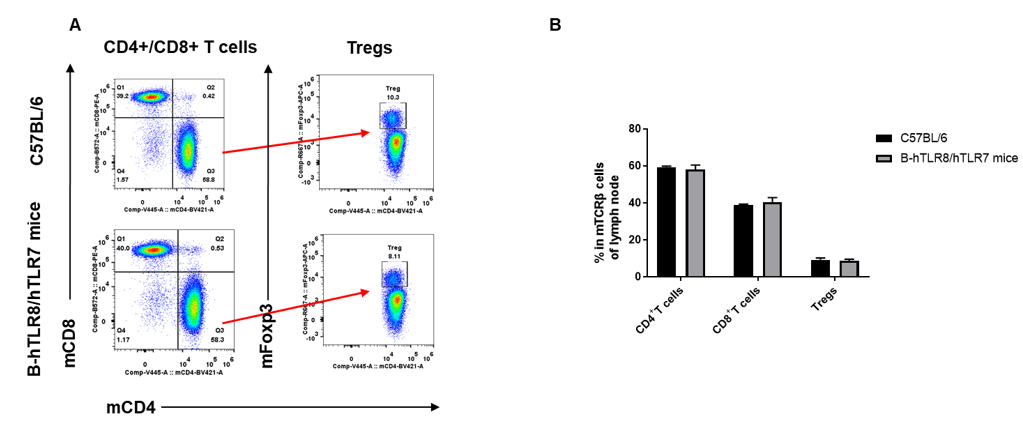
Analysis of lymph node T cell subpopulations by FACS. lymph node cells were isolated from female C57BL/6 and B-hTLR8/hTLR7 mice (n=3, 8-week-old). Flow cytometry analysis of the cells was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for TCRβ+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of Tregs in homozygous B-hTLR8/hTLR7 mice were similar to those in the C57BL/6 mice, demonstrating that TLR8 humanized does not change the overall development, differentiation or distribution of these cell types in lymph node .
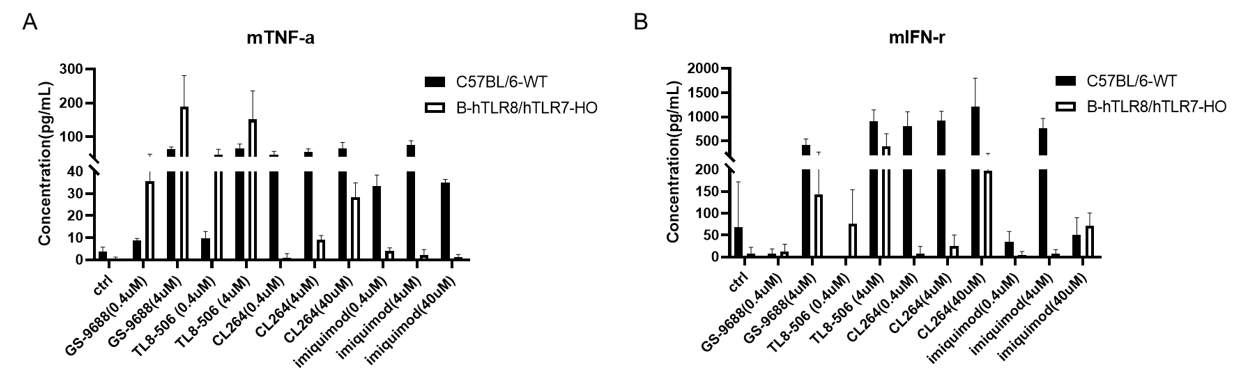
Strain specific mTNF-α and mIFN-γ expression analysis in homozygous B-hTLR8/hTLR7 mice by ELISA. Splenocytes were isolated from wild-type C57BL/6 mice (+/+) and homozygous B-hTLR8/hTLR7(H/Y, H/Y) and stimulated with TLR8/TLR7 agonist in vitro for 24 h, then cell supernatants were collected and analyzed by ELISA with species-specific mTNF-α (A) and mIFN-γ (B) ELISA kit.
Copyright © 2024 百奥赛图江苏基因生物技术有限公司. All Rights Reserved
备案号: 苏ICP备2021053911号-1
 苏公网安备:32068402320845号
网站建设:北京分形科技
苏公网安备:32068402320845号
网站建设:北京分形科技
 苏公网安备:32068402320845号
网站建设:北京分形科技
苏公网安备:32068402320845号
网站建设:北京分形科技






 010-56967680
010-56967680 info@bbctg.com.cn
info@bbctg.com.cn