中文
B-hCD3E mice(C)
| Strain Name |
BALB/cCrSlcNifdc-Cd3etm1(CD3E)Bcgen/Bcgen
|
Common Name | B-hCD3E mice(C) |
| Background | BALB/cCrSlcNifdc | Catalog number | 111876 |
|
Aliases |
CD3epsilon, IMD18, T3E, TCRE | ||
模型验证
Protein expression analysis in spleen
Analysis of main immune organs
Frequency of leukocyte subpopulations in thymus
Frequency of leukocyte subpopulations in spleen
Frequency of leukocyte subpopulations in lymph node
Frequency of leukocyte subpopulations in blood
Analysis of T cell activation stimulated with anti-CD3ε antibody in vitro
Analysis of T cell activation stimulated with anti-CD3ε antibody in vitro
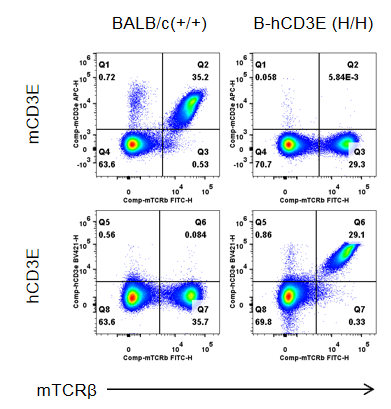
Strain specific CD3E expression analysis in wild-type BALB/c mice and homozygous humanized B-hCD3E mice(C) by flow cytometry. Splenocytes were collected from wild-type BALB/c mice (+/+) and homozygous B-hCD3E mice(C) (H/H), and analyzed by flow cytometry. Mouse CD3E was only detectable in wild-type BALB/c mice. Human CD3E was exclusively detectable in homozygous B-hCD3E mice(C), but not in wild-type BALB/c mice.
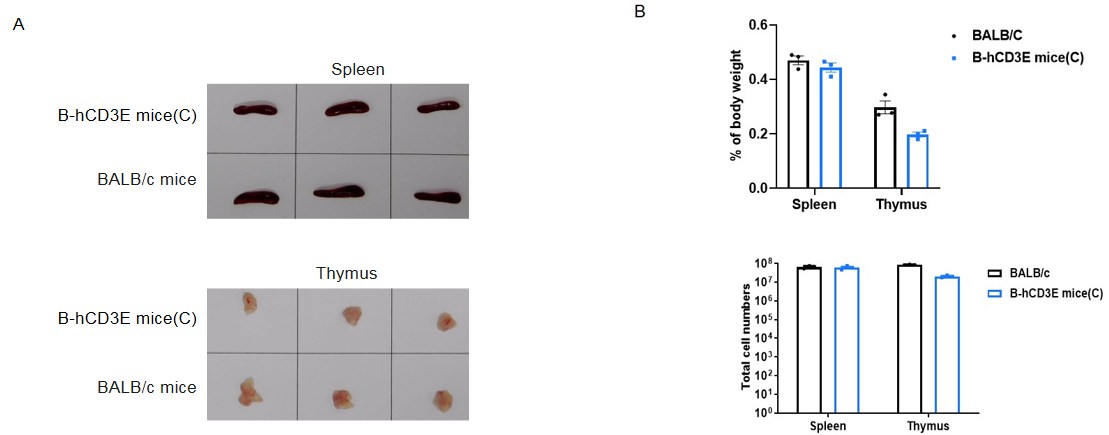
Spleen and thymus from BALB/c and B-hCD3E mice(C) (n=3) were weighed (A). Organ weight is shown as a ratio to body weight (B). Compared to BALB/c mice, there were not significantly different in spleen weight and the total number of splenocytes of B-hCD3E mice(C). The weight of thymus and the number of thymocytes in B-hCD3E mice(C) were lower than those in BALB/c mice. Data are represented as mean ±SEM.
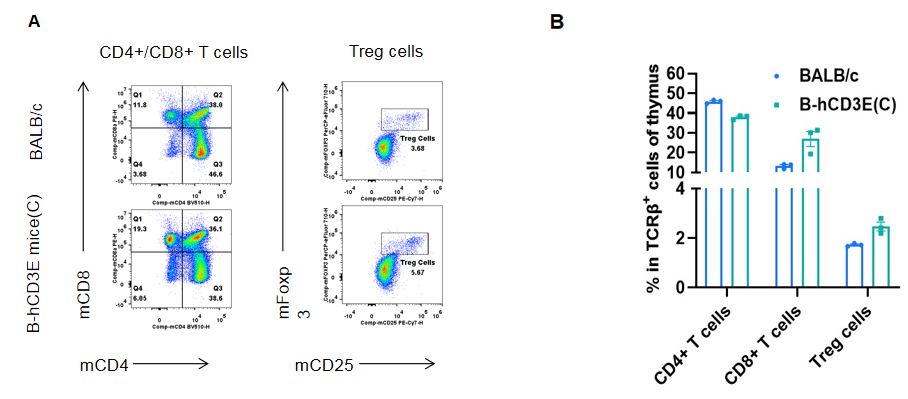
Analysis of thymus T cell subpopulations by FACS.Thymocytes were isolated from wild-type BALB/c mice and homozygous B-hCD3E mice(C) (n=3, 7-week-old). A. Representative FACS plots. B. Frequency of T cell subpopulations. Percentage of Treg cells in homozygous B-hCD3E mice(C) is similar to that in BALB/c mice. Percent of CD4+ T cells, CD8+ T cells and Treg cells in homozygous B-hCD3E mice(C) were similar to those in the BALB/c mice, demonstrating that humanization of CD3E does not change the frequency or distribution of these cell types in thymus. Values are expressed as mean ± SEM.
CD3E knockout mice lack mature T cells, and the development of thymocytes is arrested at the early CD4(-)CD8(-) stage. (DeJarnette JB, et al., Proc Natl Acad Sci U S A. 1998.)

Frequency of leukocyte subpopulations in spleen by flow cytometry. Splenocytes were isolated from wild-type BALB/c mice and homozygous B-hCD3E mice(C) (n=3, 7-week-old). A. Flow cytometry analysis of the splenocytes was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percent of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes, macrophages, CD4+ T cells, CD8+ T cells and Treg cells in homozygous B-hCD3E mice(C) were similar to those in the BALB/c mice, demonstrating that humanization of CD3E does not change the frequency or distribution of these cell types in spleen. Values are expressed as mean ± SEM.
CD3E knockout mice lack mature T cells, and the development of thymocytes is arrested at the early CD4(-)CD8(-) stage. (DeJarnette JB, et al., Proc Natl Acad Sci U S A. 1998.)
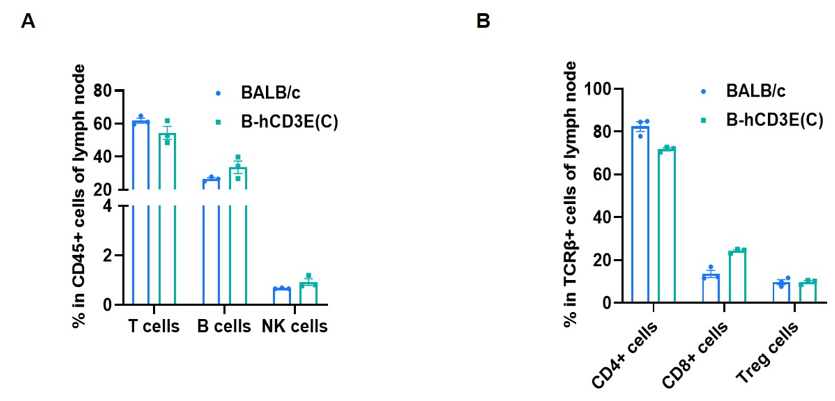
Frequency of leukocyte subpopulations in lymph node by flow cytometry. Leukocytes were isolated from wild-type BALB/c mice and homozygous B-hCD3E mice(C) (n=3, 7-week-old). A. Flow cytometry analysis of the leukocytes was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percent of T cells, B cells, NK cells, CD4+ T cells, CD8+ T cells and Treg cells in homozygous B-hCD3E mice(C) were similar to those in the BALB/c mice, demonstrating that humanization of CD3E does not change the frequency or distribution of these cell types in lymph node. Values are expressed as mean ± SEM.
CD3E knockout mice lack mature T cells, and the development of thymocytes is arrested at the early CD4(-)CD8(-) stage. (DeJarnette JB, et al., Proc Natl Acad Sci U S A. 1998.)
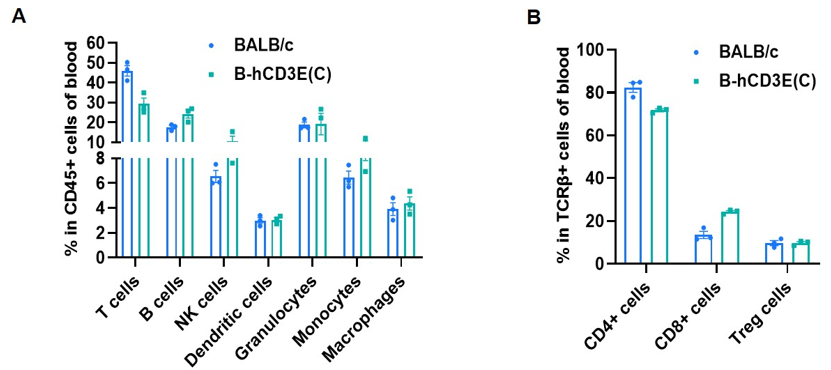
Frequency of leukocyte subpopulations in blood by flow cytometry. Blood cells were isolated from wild-type BALB/c mice and homozygous B-hCD3E mice(C) (n=3, 7-week-old). A. Flow cytometry analysis of the blood cells was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percent of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes, macrophages, CD4+ T cells, CD8+ T cells and Treg cells in homozygous B-hCD3E mice(C) were similar to those in the BALB/c mice, demonstrating that humanization of CD3E does not change the frequency or distribution of these cell types in blood. Values are expressed as mean ± SEM.
CD3E knockout mice lack mature T cells, and the development of thymocytes is arrested at the early CD4(-)CD8(-) stage. (DeJarnette JB, et al., Proc Natl Acad Sci U S A. 1998.)
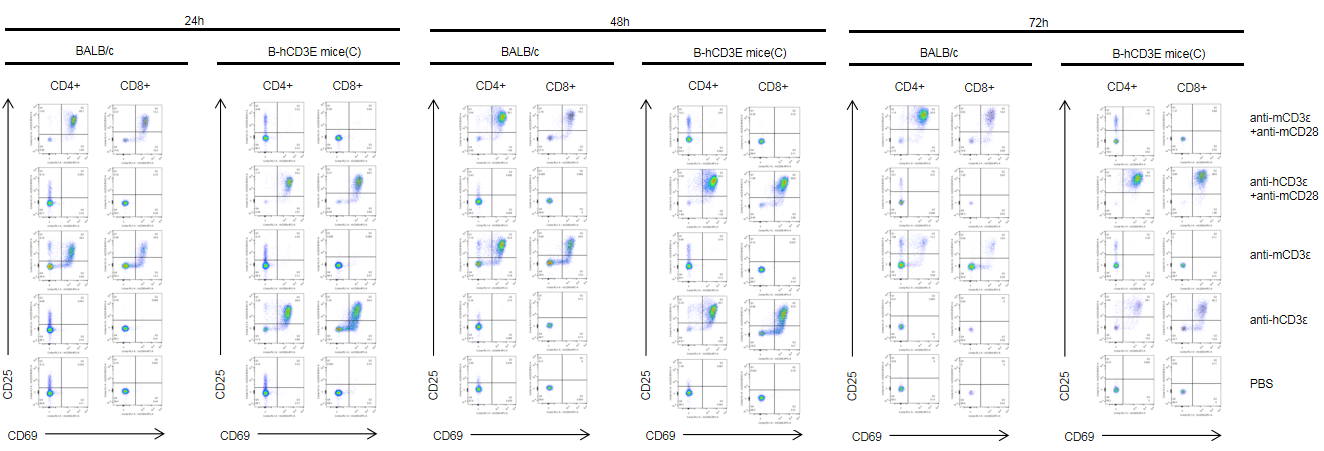
T cells (2×105) were isolated from splenocytes of BALB/c and B-hCD3E mice(C) (n=3, 8 week-old), and were incubated in the presence of anti-CD3ε antibody (2ug/ml) and anti-mCD28 antibody (5ug/ml) for 24h, 48h and 72h. T cell activation was tested by flow cytometry. T cell activation in B-hCD3E mice(C) was significantly up-regulated by anti-hCD3ε antibody alone or in combination with anti-mCD28 antibody, similar to the activation level shown in BALB/c mice treated with anti-mCD3ε antibody alone or in combination with anti-mCD28 antibody.
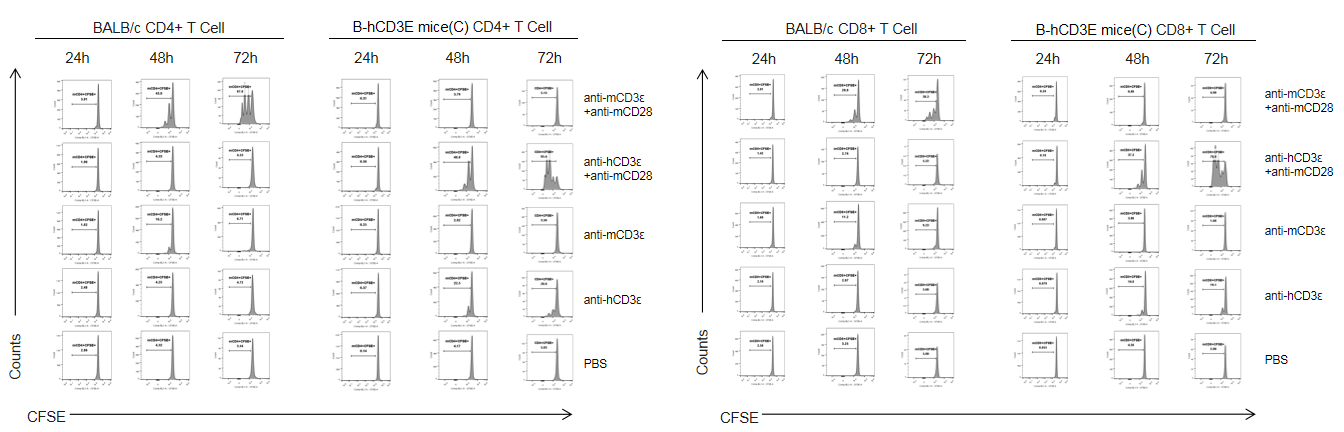
T cells (2×105) were isolated from splenocytes of BALB/c and B-hCD3E mice(C) (n=3, 8 week-old), and were incubated in the presence of anti-CD3ε antibody (2ug/ml) and anti-mCD28 antibody (5ug/ml) for 24h, 48h and 72h. T cell proliferations was tested by flow cytometry. T cell activation in B-hCD3E mice(C) was significantly increased when treated with a combination of anti-hCD3ε antibody and anti-mCD28 antibody, similar to the level of activation observed in BALB/c mice treated a combination of anti-mCD3ε antibody and anti-mCD28 antibody.
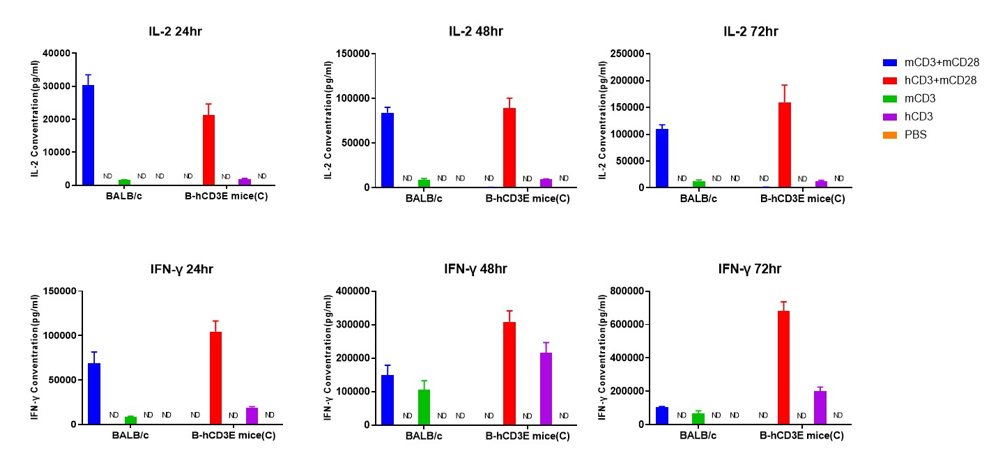
T cells (2×105) were isolated from the splenocytes of BALB/c and B-hCD3E mice(C) (n=3, 8 week-old), incubated in the presence of anti-CD3ε antibody (2ug/ml) and anti-mCD28 antibody (5ug/ml) for 24h, 48h and 72h. IFN-γ and IL-2 productions were then tested using ELISA method. When stimulated with anti-hCD3 antibody and anti-mCD28 antibody in vitro, T cells from the spleen of B-hCD3E mice (C) showed a significant increase in production of IFN-γ and IL-2, similar to the data observed in BALB/c mice. ND: not detectable.
Copyright © 2024 百奥赛图江苏基因生物技术有限公司. All Rights Reserved
备案号: 苏ICP备2021053911号-1
 苏公网安备:32068402320845号
网站建设:北京分形科技
苏公网安备:32068402320845号
网站建设:北京分形科技
 苏公网安备:32068402320845号
网站建设:北京分形科技
苏公网安备:32068402320845号
网站建设:北京分形科技






 010-56967680
010-56967680 info@bbctg.com.cn
info@bbctg.com.cn